Wastewater pollution is a pressing global issue that poses significant risks to both the environment and human health. With textile, cosmetic, ink, paper, and other industries discharging large quantities of dyes into wastewater, the need for effective treatment methods is paramount. These dyes are highly toxic and can contain potential carcinogens, making their removal essential. Unfortunately, traditional wastewater treatment methods such as sedimentation, biological oxidation, and chemical-physical treatment have proven to be ineffective in removing dyes due to their complex molecular structure and water-soluble nature. Adsorption methods using clay materials, activated carbon, and natural materials have shown promise in separating dyes from water, but the dyes still persist within the wastewater. Photocatalysts, previously considered the solution, have not been successful as they require extensive UV light treatment that consumes significant energy.
A Nano-Fueled Breakthrough
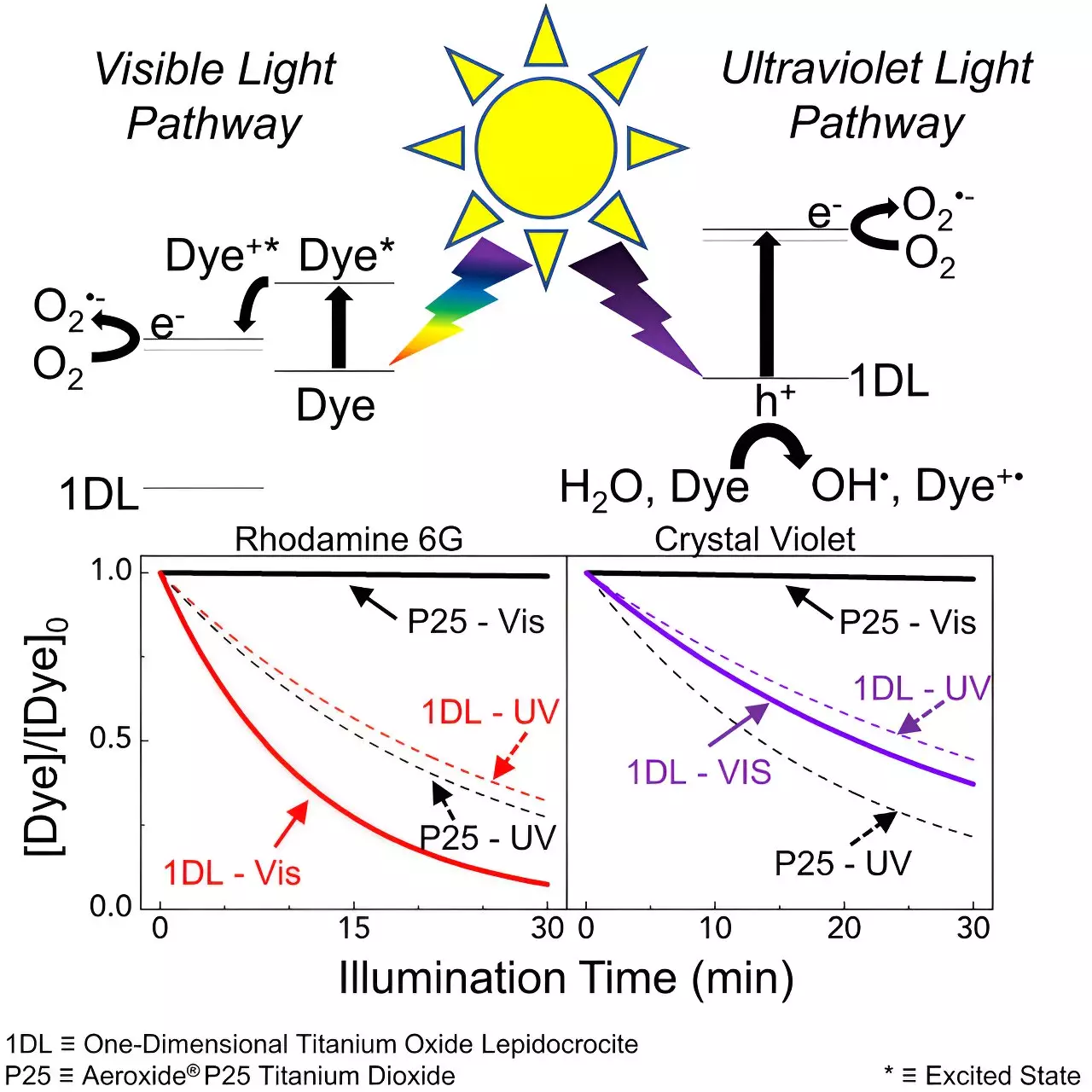
Researchers from Drexel University’s College of Engineering, led by Dr. Michel Barsoum, have made an exciting discovery that could revolutionize wastewater treatment. The team has found that a one-dimensional, lepidocrocite structured titanium oxide photocatalyst material holds the key to effective dye degradation under the visible light spectrum. This nanofilament has the ability to break down two common dye pollutants, rhodamine 6G and crystal violet, reducing their concentrations in water by 90% and 64%, respectively, in just half an hour. Unlike traditional photocatalysts, this innovative nanofilament exhibits self-sensitization behavior, making it more responsive to visible light. This opens up the possibility of using sunlight or other simulated light sources, significantly reducing energy consumption and costs associated with wastewater treatment.
The process of dye degradation begins with adsorption, where the dye adheres to the surface of the nanofilament. When exposed to visible light, the nanofilament undergoes photocatalysis, accelerating the degradation of the dye into harmless byproducts like carbon dioxide and water. The study conducted by the Drexel University team, published in the journal Matter, unveiled the key to the dye degradation and self-sensitization process. The material generates electron holes and reactive oxygen species (ROS), including hydroxyl, superoxide, and singlet oxygen radicals, which further contribute to the breakdown of the dyes.
The study conducted by Dr. Barsoum and his team involved researchers from Drexel’s College of Arts and Sciences, highlighting the interdisciplinary nature of this breakthrough. By combining expertise from engineering and materials science, the team was able to characterize the arrangement of atoms in the nanomaterial using X-ray diffraction. Scanning and transmission electron microscopy provided further insights into the structural and optical properties of the nanofilaments. To quantify dye decolorization and mineralization, ultraviolet-visible spectroscopy and chemical oxygen demand analysis were employed.
Expanding Possibilities
While this study primarily focused on the nanofilament’s effectiveness in wastewater treatment, it also opens up exciting opportunities for other fields such as solar cells and optical devices. The material’s ability to harness visible light for dye degradation suggests its potential in green fuel generation through sunlight-driven hydrogen separation.
One of the most significant findings of this study was the symbiotic relationship between the dye and the nanofilament. The dye not only catalyzed its own destruction but also sensitized the nanofilament, resulting in cleaner and less toxic water. This innovative approach holds promise for addressing water pollution at its source, effectively removing dyes without relying on additional toxins or energy-intensive processes.
Dr. Barsoum emphasizes that this breakthrough only scratches the surface of the material’s capabilities. As researchers continue exploring its potential, the nanofilament could pave the way for advancements in numerous fields, including wastewater treatment, solar energy conversion, and optical devices. By leveraging visible light, this nanofilament offers a sustainable and cost-effective solution to the persistent problem of dye pollution in wastewater.
The discovery of this one-dimensional, lepidocrocite structured titanium oxide nanofilament marks a significant advancement in the field of wastewater treatment. Its ability to break down common dye pollutants under visible light not only offers a more efficient solution but also reduces energy consumption and costs. As the world grapples with the consequences of industrial pollution, innovative technologies like this nanofilament provide a glimmer of hope for a cleaner and more sustainable future.

Leave a Reply