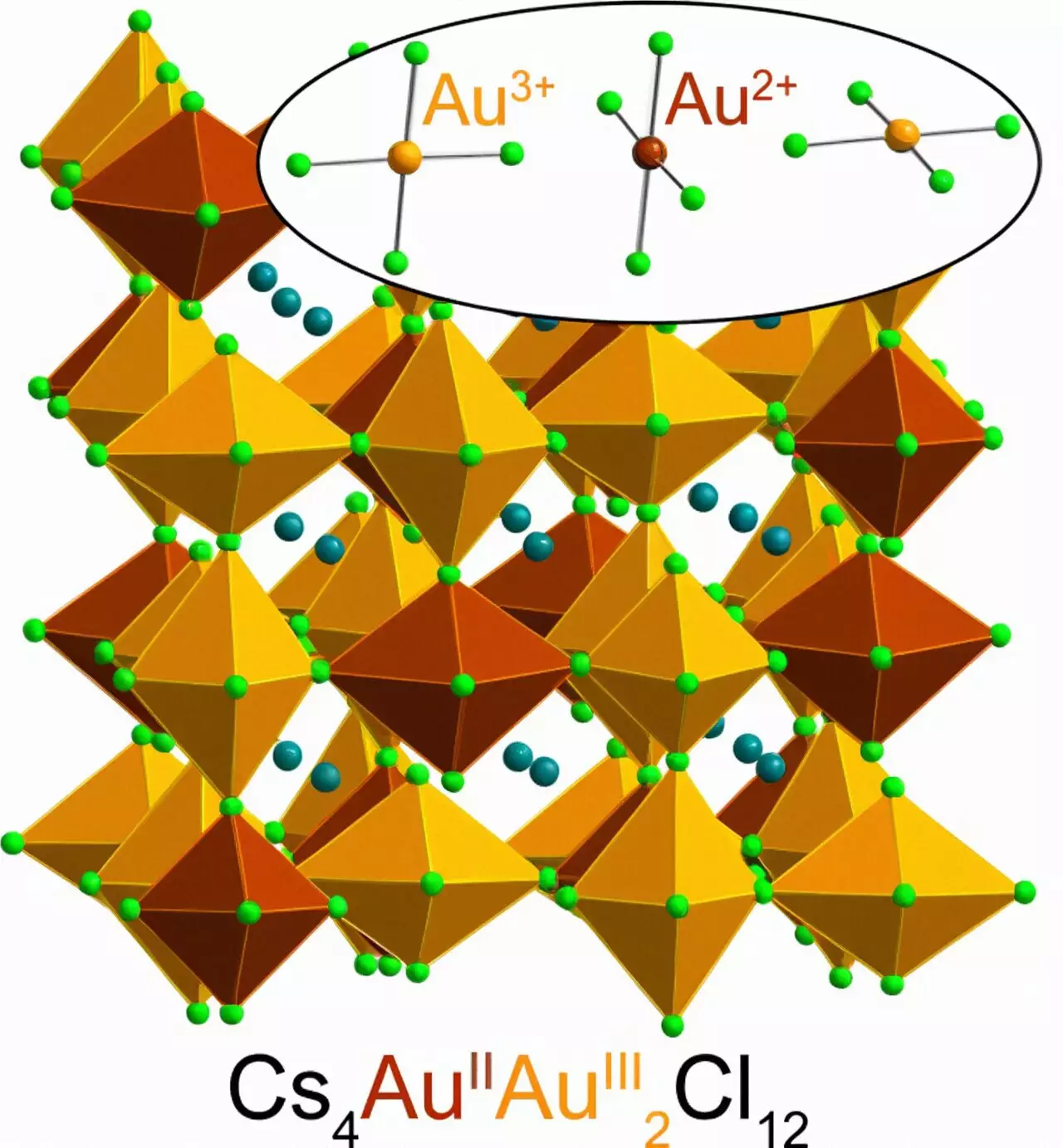
For the first time, researchers at Stanford University have successfully created and stabilized a rare form of gold, known as Au2+. This discovery was made possible through the use of a halide perovskite—a type of crystalline material that shows great promise in various applications, including solar cells, light sources, and electronics components. What makes this achievement even more remarkable is that the process of creating this unique form of gold is quick and straightforward, using readily available materials at room temperature.
Surprising and Unexpected Results
Professor Hemamala Karunadasa, the senior author of the study, expressed her initial disbelief at the ability to synthesize a stable material containing Au2+. The discovery opens up new possibilities for understanding the properties and applications of heavy metals with unpaired electrons, such as gold. It is worth noting that the gold atoms in the perovskite bear strong similarities to the copper atoms found in high-temperature superconductors. Heavy atoms like Au2+ also exhibit cool magnetic effects not seen in lighter atoms, further adding to the significance of this finding.
Gold has long been valued for its scarcity, malleability, and resistance to chemical reactions. These qualities make it perfect for jewelry and coins that maintain their appearance over time. The distinctively rich golden color of gold is a result of its electron configuration and relativistic effects. As predicted by Albert Einstein’s theory of relativity, particles that move at high speeds become heavier. In the case of gold, the heavy atomic nuclei exert immense positive charge, causing the electrons to whirl around the nucleus at tremendous speeds. This rearrangement of electrons leads to gold’s absorption of blue light and its characteristic yellow appearance.
The Challenges of Au2+
Au2+ is incredibly rare due to the specific electron arrangement of gold atoms. In its pure state, gold naturally occurs as Au1+ and Au3+, losing one or three electrons, respectively, and not forming Au2+. Au2+ represents a net positive charge resulting from the loss of two negatively charged electrons. This rarity is why the synthesis of a stable Au2+ perovskite is such a significant achievement.
The Synthesis Process
Au2+ perovskite was discovered by accident when lead author Kurt Lindquist mixed cesium chloride and Au3+-chloride in water, adding hydrochloric acid and vitamin C to the solution. The reaction involved vitamin C donating an electron to Au3+, thereby forming Au2+. Interestingly, Au2+ is stable in the solid perovskite but not in solution. The simplicity of the synthesis process adds to the excitement, as this material can be made using basic ingredients in just five minutes at room temperature. The resulting powder is dark green, almost black, and surprisingly heavy due to its gold content.
Unveiling the Secrets of Au2+
Various spectroscopy and X-ray diffraction tests were conducted to investigate the light absorption and crystal structure of the Au2+ perovskite. Research groups at Stanford, led by Professor Young Lee in physics and Professor Edward Solomon in chemistry, contributed to uncovering the behavior of Au2+. These experiments confirmed the presence of Au2+ in the perovskite and added a new chapter to the ongoing story of gold perovskites, originated by Linus Pauling, a Nobel laureate in Chemistry. Coincidentally, Pauling also studied the structure of vitamin C, which played a significant role in stabilizing the elusive Au2+ perovskite.
With this breakthrough, Professor Karunadasa, Lindquist, and their colleagues plan to further study and modify the chemistry of the Au2+ perovskite. Their hope is to explore potential applications that require magnetism and conductivity, leveraging the electron hopping between Au2+ and Au3+ within the perovskite structure. The discovery of the Au2+ perovskite offers exciting opportunities for advancements in various fields and lays the groundwork for future innovations.
The creation and stabilization of Au2+ perovskite represent a remarkable achievement in the field of chemistry. This groundbreaking discovery not only sheds light on the properties of gold but also opens up new avenues for the development of materials with unique magnetic and conductive properties. The simplicity of the synthesis process using readily available ingredients at room temperature adds to its appeal and potential for widespread application. As researchers continue to explore the possibilities offered by Au2+ perovskite, we can expect intriguing advancements in various fields, including solar energy, electronics, and materials science.

Leave a Reply