Organic synthesis is a fascinating art that involves creating molecules for various purposes, from pharmaceuticals to materials for cutting-edge technologies. This process can be compared to playing with microscopic LEGO bricks, where chemists connect simple building blocks to form complex molecules. However, one crucial challenge in this puzzle is bonding two carbon atoms together. Just like LEGO bricks, carbon atoms need to fit together to combine easily. Unfortunately, the most reactive carbon atoms usually carry a positive charge, making them incompatible with each other. In the early days of organic chemistry, researchers found a clever workaround to this issue by using organometallic compounds. By bonding carbon to metals like zinc or magnesium, they could switch the charge of the carbon atom from positive to negative, enabling the creation of suitable combinations with other organic molecules. This breakthrough opened up a vast playground for chemical creativity.
Victor Grignard, a French chemist, made one of the most impactful discoveries in the field of organic synthesis. He found a method for creating organic derivatives of readily available magnesium, a technique that revolutionized the field. Grignard’s discovery was so significant that he was awarded the Nobel Prize in 1912. However, the Grignard method has its downsides. The highly reactive metal-containing molecules are unstable and easily break down when exposed to moisture or air, making industrial applications challenging.
The story takes an ironic twist when we look at Philip Barbier, Grignard’s scientific teacher. Barbier was the pioneer of organometallic chemistry but never received the same recognition as his student. He attempted to join carbon atoms using a similar method, but with unsatisfactory results. Despite his efforts, Barbier tasked Grignard with perfecting his method, which ultimately led to the Nobel-winning discovery.
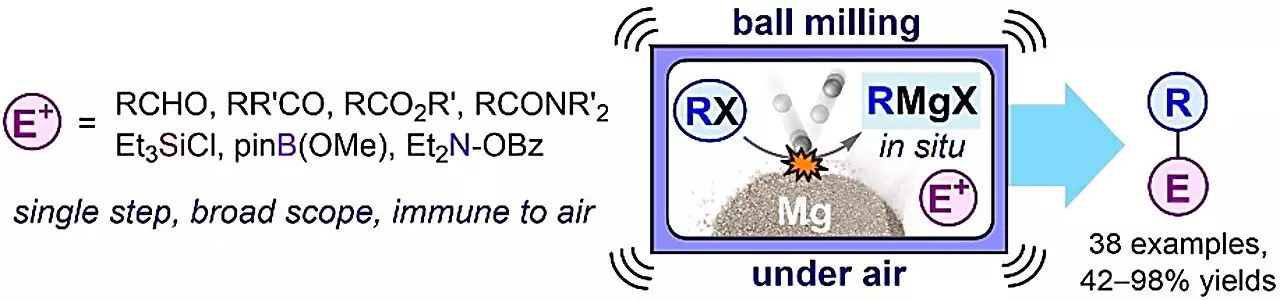
More than a century later, a group of chemists from TalTech’s supramolecular chemistry research group, led by Prof. Riina Aav and Dr. Dzmitry Kananovich, has revived and improved the Barbier method. Instead of using traditional organic solvents and mixing chemicals with magnesium metal, they discovered that milling them together, without a solvent, in a device called a shaker mill, results in remarkable improvements in efficiency and environmental friendliness. This exciting development brings the Barbier method back into the spotlight, making it as effective as the famous Grignard method. Their findings have been recently published in Angewandte Chemie International Edition.
Mechanochemistry: An Ancient Technique Revisited
The technique used by the researchers is called mechanochemistry, which has been known since ancient times but was abandoned by the scientific community in favor of more traditional solution-based chemistry. Mechanochemical devices are similar to coffee grinders, where chemical reactions occur through blending, milling, and grinding of solid substances rather than mixing solutions. This old technique is gaining traction again due to its environmental and safety benefits. Mechanochemistry eliminates the need for dangerous organic solvents, which pose serious threats to both human health and the environment.
The preparation of organometallic compounds is a particularly exciting area of focus in chemistry, with many esteemed research groups exploring this direction. TalTech’s team revisited the original idea of Barbier, making the use of organometallic compounds even more straightforward and convenient. The new method demonstrates exceptional resistance to air and certain weak acids, which are known to disrupt traditional approaches like the Grignard technique. As the organometallic compounds only exist briefly as intermediates in this process, they can keep reacting and create stable end products. This discovery holds great promise for revolutionizing the production of numerous valuable substances. It has the potential to simplify, enhance safety, and improve the environmental friendliness of manufacturing processes, especially in industries such as pharmaceuticals.
TalTech’s team is not stopping at the rediscovery and improvement of mechanochemistry. They are now collaborating with researchers from eleven other European countries on the IMPACTIVE project, which aims to transform the pharmaceutical sector through mechanochemical production methods. This innovative project could be the key to unlocking new opportunities in the chemical industry, making it safer and more sustainable for generations to come. It is a perfect blend of the old and the new, offering the promise of a brighter future.

Leave a Reply