Fluorinated gases, known for their hazardous properties, have long been a challenge when it comes to handling and usage. Not only are they flammable and toxic, but they also contribute to the depletion of the ozone layer upon release into the atmosphere. Recognizing the need for a safer and more efficient method for handling these gases, a team of chemists from Cornell University, the Korea Institute of Science and Technology, and Southern Methodist University have made significant strides in this area. Their groundbreaking research, published in the journal Science, demonstrates the potential of metal organic frameworks (MOFs) to provide a safer and cleaner solution.
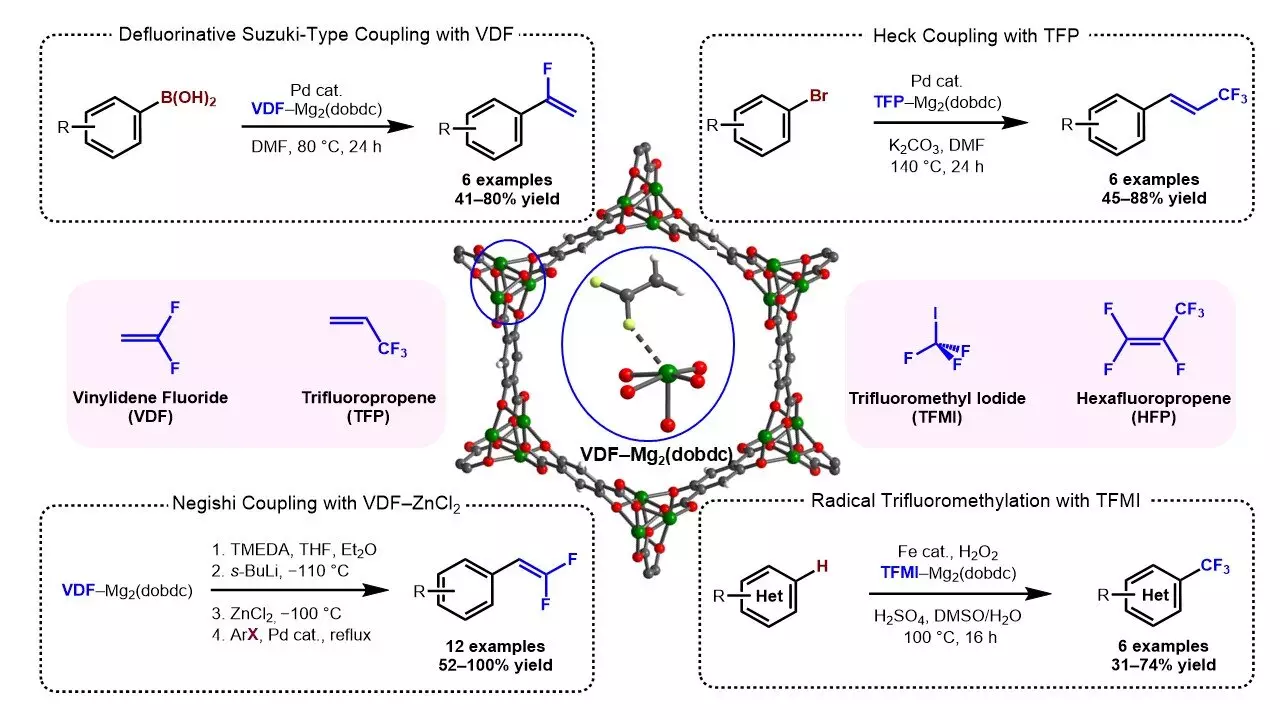
When approaching the issue of handling fluorinated gases, the research team understood the requirements of a suitable material. It needed to be porous, allowing for the absorption and storage of gases. Additionally, the material had to effectively interact with the gases, preventing any leakages, while remaining stable over extended periods. To identify the optimal material for this purpose, the team tested the uptake of vinylidene fluoride in 12 different MOFs. MOFs are compounds comprising coordinated organic ligands with various structures.
Among the MOFs tested, one specific type called Mg2 stood out as an ideal candidate. Mg2 demonstrated both high gas sorption capacity and strong interactions with multiple fluorides. Furthermore, this material exhibited impressive durability and stability. It was able to remain viable even after being left on a benchtop for an entire week. The researchers also discovered that any gas contained within Mg2 could be easily released when necessary by immersing it in a solvent.
The creation of gas-MOF reagents was another essential finding of the study. By using Mg2 as a containment material, the researchers successfully held a gas for up to a week without any leakage. To further prolong the storage period, they embedded the gas-MOF reagents in wax, which enabled safe and effective gas retention for up to two months. It is worth noting that accessing the gases stored in the MOFs simply required applying sonication, a relatively straightforward process.
The use of metal organic frameworks to handle fluorinated gases has significant implications for a range of industries and applications. By eliminating the risk of leaks and providing a safer storage method, MOFs could revolutionize the transportation and usage of these hazardous gases. For instance, industries requiring fluorinated gases for various processes, such as refrigeration and electronics manufacturing, could benefit greatly from this breakthrough. Additionally, the potential long-term containment offered by embedding gas-MOF reagents in wax opens up possibilities in areas like gas storage and transportation.
The research conducted by the team of chemists from Cornell University, the Korea Institute of Science and Technology, and Southern Methodist University showcases the potential of metal organic frameworks for the safer handling of fluorinated gases. Their discovery of Mg2 as an ideal candidate for gas sorption and containment represents a significant advancement in the field. With the ability to hold gases securely without leakage, and the potential for long-term storage, MOFs could pave the way for a more secure and sustainable future in handling hazardous substances.

Leave a Reply